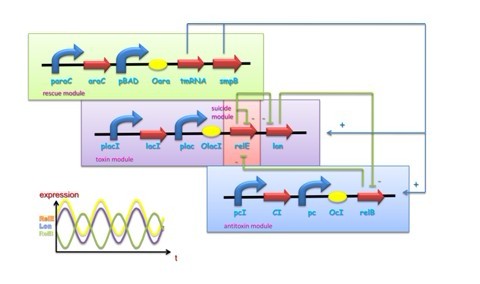
Bacteria and at least some archaea harbor ‘suicide systems’, the existence of which is a mystery to those who hear about them for the first time. These systems consist of a lethal toxin that can be neutralized by an antitoxin. The production of antitoxin, which is labile, must at least parallel that of the toxin, which is usually stable, to circumvent the induction of cell poison. To achieve this, both proteins are encoded within a single operon. The toxins seem to target specific, essential cellular processes. Perhaps the best characterized of these system is relBE system, the genes of which are present on the Escherichia coli chromosome. The relE toxin is an RNase that preferentially cleaves mRNAs bound to the ribosome at the second position of stop codons. Stop codons not only signal the end of the protein coding sequence but also serve as the binding site for release factors, which promote release of the nascent polypeptide and facilitate recycling of ribosomes for further rounds of translation. Thus truncated mRNA by cleavage of relE lacks appropriate termination signals, which causes the accumulation of stalled ribosomes and these mRNAs are unable to promote release factor binding, nascent polypeptide release, and ribosome recycling.
2009 Team Wiki:http://2009.igem.org/Team:SJTU-BioX-Shanghai